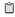Technical Protocols
Flow cytometric analysis of mouse platelet surface glycoproteins
- Preparation of diluted or washed whole blood
- Preparation of washed mouse platelets
- Single-color analysis of platelet surface glycoprotein expression
- Two-color analysis of platelet activation
Immunohistochemistry (with acetone-fixed frozen sections)
Immunoblot (Western Blot Analysis)
Flow cytometric analysis of mouse platelet surface glycoproteins
Buffers and reagents
Tris buffered saline/Heparin (TBS/Hep): 20 mM Tris/HCl; 137 mM NaCl; pH 7.3; containing 20 U/ml Heparin.
Phosphate buffered saline (PBS): 137 mM NaCl; 2.7 mM KCl; 1.5 mM KH2PO4; 8 mM Na2HPO4; pH 7.14.
Tyrode’s Buffer (modified): 134 mM NaCl; 0.34 mM Na2HPO4; 2.9 mM KCl; 12 mM NaHCO3; 20 mM Hepes; pH 7.0; 5 mM glucose; 0.35% (w/v) bovine serum albumin
Apyrase: 10 U/ml stock in H2Obidest (stored at -20°C)
Prostacyclin (Prostaglandin 2, PGI2): 1 mM stock in H2Obidest (stored at -20°C)
CaCl2: 1 M stock in H2Obidest
Preparation of diluted or washed whole blood
- Take 50 µl blood with a heparinized glass capillary (e.g. ringcap) from the retro-orbital plexus into 1.5 ml tubes containing 200 µl of TBS/Heparin (20 U/ml).
- Dilute in 1 ml of Tyrode´s buffer and use for flow cytometric analysis.
- To prepare washed blood, centrifuge the diluted blood at 900 x g for 5 min, remove the supernatant and resuspend the pellet in 1.25 ml Tyrode´s buffer.
- Add 1 mM CaCl2 directly before the start of the experiment.
Note: For thrombin-induced platelet activation, plasma has to be removed to prevent anti-thrombin activity
Preparation of washed mouse platelets
The preparation of washed platelets is a more time consuming method, that requires a larger amount of blood, but yields a platelet preparation that can be used for several hours.
- Collect 0.5 - 1 ml blood with 1.5 cm glass capillaries from the retro-orbital plexus into a 1.5 ml tube containing 200 µl of TBS/Heparin (20 U/ml).
- Centrifuge the sample for 5 min at 500 x g and transfer the platelet rich plasma (PRP) into a new tube. For best recovery of platelets, take the complete white phase including some red blood cells.
- Centrifuge the platelet suspension for 8 min at 300 x g, and transfer the PRP without any red blood cells into a new tube.
- Add 0.5 µM prostacyclin (PGI2) and centrifuge at 1,300 x g for 5 min.
- Resuspend the platelet pellet in 1 ml Tyrode’s buffer, add 0.02 U/ml apyrase and 0.5 µM prostacyclin, incubate for 5 min at 37°C, and centrifuge for 5 min at 1300 x g.
- Repeat step 5 and resuspend the platelet pellet in 0.5 ml Tyrode’s buffer, add 0.02 U/ml of apyrase and incubate for 30 min at 37°C.
Single color analysis of platelet surface glycoproteins
- Combine 5 µl of specific or negative control antibodies and 25 µl diluted whole or washed blood in the assay tube and vortex mix for 1-2 seconds.
- Incubate for 15 min at room temperature.
- Stop reaction by addition of 400 µl PBS and analyze within 30 min.
Samples were incubated with FITC-conjugated control IgG (black line), anti-GPVI, or anti-GPV as described. Platelets were gated by forward (FSC) / side scatter (SSC) characteristics. Fluorescence 1 (FL1) intensity of the gated (R1) platelets is shown in separate histograms. RBC: red blood cells.
Two color analysis of platelet activation
- Add 5 µl specific or negative control antibodies to the assay tube together with 5 µl of a pan-platelet marker.
- At this point, any additional reagents (e.g. inhibitors) are added in small volumes (< 5 µl).
- Add 26 µl diluted whole or washed blood or washed platelets (1 million) and vortex mix for 1-2 seconds.
- Incubate for 15 min at room temperature. Stop reaction by addition of 400 µl PBS and analyze within 30 min.
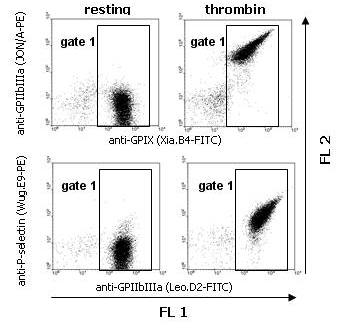
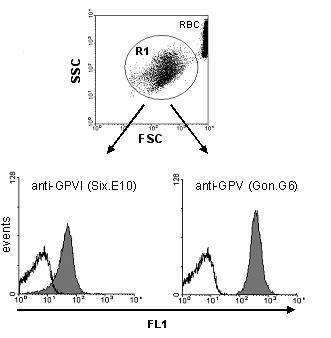
Washed mouse platelets were incubated with PBS (resting) or thrombin (0.1 U/ml) in combination with both FITC- and PE-conjugated mAbs. Platelets were gated by expression of GPIX or GPIIbIIIa (FL1; gate 1).
Immunohistochemistry (with acetone-fixed frozen sections)
Please note: It is not recommended to perform immune staining on (para-)formaldehyde-fixed samples, since most rat antibodies yield only poor results under these conditions on mouse tissues.
- Mount sections from frozen tissue on slides and fix in ice cold 100% acetone for 20 min at 4°C. Dry samples over night at RT.
- To minimize reagent volumes, a hydrophobic ring may be drawn with a water repellent pen around the tissue section on the slide. During all following procedures, this encircled area should not dry up. Therefore, it is recommended to perform incubation steps in a humid box.
- Incubate slides in PBS for 20 min.
- When using peroxidase-conjugated secondary antibodies, incubate slides with 0.03% H2O2 for 10 min at RT to inhibit endogenous peroxidase activity. Rinse slides three times in PBS for 5 min each wash.
- Incubate slides for 1 hour at RT in blocking solution (serum from host species of secondary antibody to be used), diluted 1:10 or 1:100 in PBS.
- Cover slides with primary antibody (2-10 µg/ml) in blocking solution and incubate for 2 h at RT.
- Blot excess liquid from slides and rinse three times in PBS for 5 min each wash.
- Cover slides with fluorophore- or peroxidase-conjugated secondary antibody (e.g. affinity purified donkey anti-rat IgG-FITC or -HRP) diluted in blocking solution according to the manufacturer’s instructions. Incubate for 1 h at RT.
- Blot excess liquid from slides and rinse three times in PBS for 5 min each wash.
- a) Fluorescently stained samples can be directly analyzed by fluorescence microscopy.
- b) For visualization of peroxidase-labeled structures, cover slides with freshly prepared 3-amino-9-ethylcarbazole (AEC) substrate and incubate until red staining is visible under the microscope, time can vary between 5 and 30 min. Stop reaction by rinsing with deionized water before orange background staining occurs. For counterstaining, incubate samples for 30-240 s with hematoxylin and rinse slides for 20 min with running warm water. Mount sections with aqueous embedding solution.
Immunoprecipitation
Buffers and Reagents
PBS/EDTA: 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.0 mM Na2HPO4 x 2 H2O, pH 7.3; 5 mM EDTA
IP buffer: 40 mM Tris/HCl, 0.3 M NaCl, 1 mM EDTA, 2 mM PMSF, 10 µg/ml Leupeptin, 10 µg/ml Aprotinin, 1 µg/ml Pepstatin
Igepal: 10% stock in H2Obidest
2x SDS-sample buffer: 10% beta-mercaptoethanol (for reducing buffer), 10% Tris buffer (1.25 M), 20% Glycerin, 4% SDS, 0.02% Bromophenolblue
- Wash mouse platelets or cells (10 million per immunoprecipitation) 3 x in1 ml PBS/EDTA (5 min, 1,300 x g) and resuspend in IP-buffer.
- Lyse platelets by addition of 1% Igepal for 15 – 20 min at RT.
- To remove cell debris, spin 5 min at 10,000 x g and transfer supernatant to a new cup.
- Preclear by adding 20 µl of washed protein G agarose (PGA). Incubate with rotation for at least 3 h at 4°C.
- Centrifuge the samples for 5 min at 3,000 x g. Fill supernatants into new tubes, add the antibody at a concentration given in the data sheet (usually between 2 and 5 µg/ml), and after 30 min add 25 µl of washed PGA. Incubate with rotation for at least 2 h at 4°C, preferably over night.
- To wash the precipitate, centrifuge the samples for 1 min at 3,000 x g. Add 1 ml of 1 x IP buffer containing 1% Igepal to the PGA pellet. Centrifuge for 1 min at 3,000 x g.
- Wash the PGA pellet twice in plain IP-buffer (1 min at 3,000 x g).
- Resuspend the PGA pellet in 25 µl of 1x SDS-sample buffer (reducing or non-reducing) and boil for 5 min. The samples can then be frozen (-20°C) for later use, or SDS-PAGE and subsequent immunoblot analysis can be performed.
Immunoblot (Western Blot Analysis)
Buffers and reagents
Lysis buffer: 40 mM Tris/HCl, 0.3 M NaCl, 1 mM EDTA, 0.05% NaN3, 1% Igepal
PBS: 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.0 mM Na2HPO4 x 2H2O, pH 7.3
PBS-5% milk: PBS containing 5% non-fat dry milk
PBS/T: PBS containing 0.1% Tween 20
PBS/T-1% milk: PBS/T containing 1% non-fat dry milk
All steps can be performed at room temperature:
- Prepare lysate of mouse platelets or cells in lysis buffer and incubate with (2x) SDS sample buffer for 5 min at 95°C.
- Separate proteins by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or nonreducing conditions, as indicated in the respective data sheet, and transfer the proteins to PVDF membrane.
- Block the membrane with PBS-5% milk for 1 h with agitation.
- Incubate the membrane with 2-5 µg/ml of primary antibody in PBS/T-1% milk for 1 h with agitation.
- Rinse the membrane twice with PBS/T followed by two 30 min washing periods in PBS/T. Washing procedure may be reduced to three periods of 10 min each, but that should be determined individually.
- Incubate with secondary antibody (HRP-conjugated anti-rat Ig) in PBS/T-1% milk for 45–60 min with agitation.
- Rinse the membrane twice with PBS/T followed by two 30 min washing periods in PBS/T.
- Visualize bound antibody with ECL (enhanced chemiluminiscence).